Page 56 - A&A Patents&Design Rewind-2016
P. 56
A CASE STUDY- SECTION 3(C)he Patent Office, in particular the Chennai Patent Office is on a spree of refusing patent applications on the ground of
Section 3(c). Section 3(c) of the Indian Patents Act bars from patentability the mere discovery of a scientific principle
Tor the formulation of an abstract theory or discovery of any living thing or non-living substances occurring in nature.
Approximately 15 applications/ claims objected under Section 3(c) have been refused. A majority of these applications relate to
Biotechnological inventions, and in particular antibodies. The Controllers have relied on sequence listing to see the source of origin
of the sequence claimed/source of origin of the sequence which has been used to define the claimed entity. The applications are
not getting granted for the reason of being a discovery of substance occurring in nature, if the sequence is not a recombinant or
an artificial sequence. Some of the cases refused are as follows:
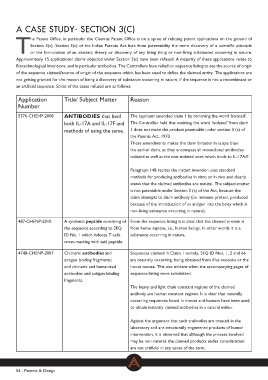
Application Title/ Subject Matter Reason
Number
ANTIBODIES that bind The applicant amended claim 1 by removing the word ‘isolated’.
5376-CHENP-2008 both IL-17A and IL-17F and The Controller held that omitting the word ‘isolated’ from claim
1 does not make the product patentable under section 3 (c) of
methods of using the same. the Patents Act, 1970.
These amendments makes the claim broader in scope than
the earlier claim, as they encompass all monoclonal antibodies
isolated as well as the non-isolated ones which binds to IL-17A/F.
Paragraph 148 recites the instant invention uses standard
methods for producing antibodies in vitro or in vivo and clearly
states that the claimed antibodies are natural. The subject-matter
is not patentable under Section 3 (c) of the Act, because the
claim attempts to claim antibody (i.e. immune protein, produced
because of the introduction of an antigen into the body which is
non-living substance occurring in nature).
487-CHENP-2010 A synthetic peptide consisting of From the sequence listing it is clear that the claimed protein is
4748-CHENP-2007 the sequence according to SEQ from homo sapiens, i.e., human beings. In other words it is a
ID No. 1 which induces T cells substance occurring in nature.
cross-reacting with said peptide.
Chimeric antibodies and Sequences claimed in Claim 1 namely, SEQ ID Nos. 1, 2 and 66
antigen-binding fragments; are naturally occurring, being obtained from Mus musculus or the
and chimeric and humanized house mouse. This was evident when the accompanying pages of
antibodies and antigen-binding sequence listing were scrutinized.
fragments.
The heavy and light chain constant regions of the claimed
antibody are human constant regions. It is clear that naturally
occurring sequences found in mouse and humans have been used
to obtain instantly claimed antibodies in a natural milieu.
Against the argument that such antibodies are created in the
laboratory and are structurally engineered products of human
intervention, it is observed that although the process involved
may be non-natural, the claimed products under consideration
are not artificial in any sense of the term.
56 | Patents & Design